Abstract
Introduction: Between 2011 and 2016, 16 novel products were approved by the Food and Drug Administration (FDA) for malignant hematology indications. The FDA has created 4 review programs to expedite the development of new drugs that address unmet medical needs in the treatment of a serious or life-threatening conditions: Fast Track designation, Breakthrough Therapy designation, Priority Review designation, and Accelerated Approval. The data used to support Accelerated Approval may rely on surrogate or intermediate clinical endpoints that have been determined to reasonably predict clinical benefit, but postapproval confirmatory trials are often required. We aimed to examine the fulfillment of postmarketing commitments associated with novel drugs granted Accelerated Approval for malignant hematology indications.
Methods: We reviewed FDA yearly approval reports between 2011-2016 to identify novel products for malignant hematology conditions, as well as use of expedited programs. FDA's Postmarketing Requirements and Commitments database was subsequently searched for products with Accelerated Approval. Moreover, the www.clinicaltrials.gov website was searched for postmarketing study status. Drug approval packages were then reviewed for further information on label changes.
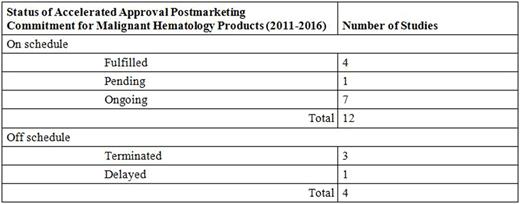
Results: Compared to products approved by the FDA for all other conditions, a significantly greater proportion of products approved for malignant hematology indications used any of the expedited programs (94% versus 56%), including Accelerated Approval (69% versus 10%). Of the 11 malignant hematology products granted Accelerated Approval, 9 were required to fulfill postmarketing commitments to collect additional clinical data, with 16 total clinical studies mandated. Significant safety issues were identified for 2 products in 3 studies (2 terminated [Zydelig], 1 fulfilled [Iclusig]), which resulted in changes to labeling for both drugs. One of the products (Iclusig) was temporarily pulled from the market, with the FDA requiring further studies, and a risk evaluation and mitigation strategy. Another postmarketing commitment study (for Beleodaq) is delayed; 1 study (for Blincyto) was terminated prematurely for nonsafety related reasons. In total, only 4 of the 16 postmarketing commitment studies have been fulfilled (see Table).
Conclusions: Nearly all novel drug products for malignant hematology conditions approved by the FDA in the past 6 years utilized an expedited program. While most products were granted Accelerated Approval, only a fraction (25%) of the associated postmarketing commitments have been fulfilled. Serious safety concerns were identified in 2 of the 9 products with postmarketing commitments following Accelerated Approval. We argue that critical assessment of the FDA Accelerated Approval program is warranted as newer drugs continue to enter the market.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal